A 61-year-old grandmother from New South Wales is among the first Australians to receive a newly approved medication for early Alzheimer’s disease.
The drug, called Kisunla or Donanemab, is the first new medicine for early Alzheimer’s disease in 25 years.
“Donanemab and the anti-amyloid class of medications is really a breakthrough, a new age in Alzheimer’s treatment,” neurologist Dr Rowena Mobbs said.
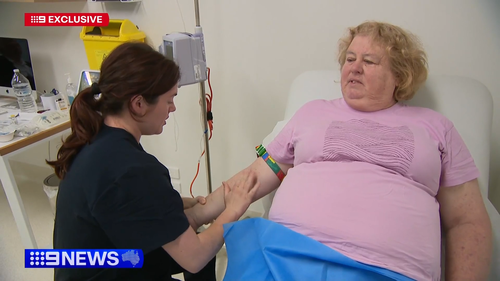
“We now have something to slow the condition after 100 years of research.”
Jenny Quiring, whose nursing career was curtailed by early symptoms of Alzheimer’s, has just received the drug.
“It’s probably the first little bit of hope,” husband James Quiring said.
The treatment will give Jenny a chance to spend more quality time with her husband of 41 years, their seven children and 15 grandchildren.
“We’d like to see them grow up and be happy,” she said.
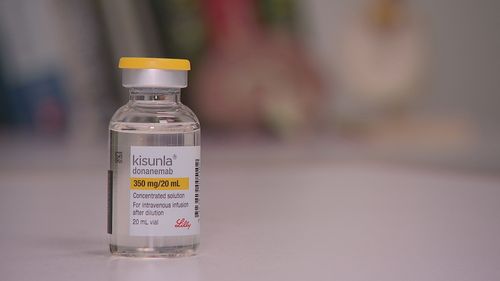
The half-hour monthly infusions will be costly, with the required scans and an 18-month course of treatment costing up to $100,000.
But a government advisory committee is expected to decide on whether Donanemab will be listed on the Pharmaceutical Benefits Scheme (PBS) in the coming weeks.
“It should be on the PBS, it’s definitely worth it,” Jenny said.
The drug’s manufacturer has released longer-term data indicating that the benefits of the treatment increase over three years. The data also suggests that earlier intervention reduces the risk of disease progression by 27 per cent compared to delaying treatment.





